Before going into the survey, it is a good idea to have a brief review of elerGreen.
Multipurpose Electrochemical Polymer Production
Background
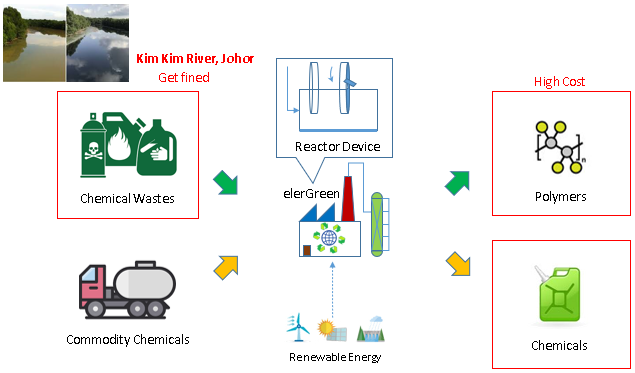
Chemical industry is carbon-emission-intense, where fossil fuels are being combusted to produced heat to drive chemical reactions. While carbon-free renewable electricity has been gaining traction, the chemical industry is still primarily driven by heat from fossil fuel combustion. Furthermore, conventional polymer and chemical industry faces high cost from heating, pressurizing, catalysts and reagents to drive the reaction. On the other hand, the traditional chemical industry inevitably produces chemical wastes, while being harmful when emitted to the environment, is equally economically damaging to the chemical industry, such as resulting local emergency and punitive fines.
Electrochemical production of polymers from chemical wastes offers the means to fill the gap for carbon-free renewable energy to be applied in chemical industry, to eliminate the use of fossil fuels. The method offers an excellent way to drastically reduce the capital and operating cost of polymers and chemicals production.
Solutions
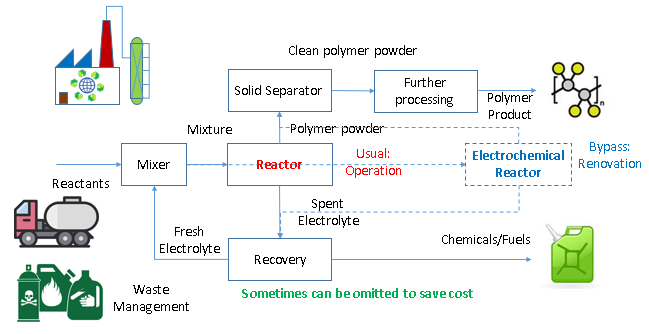
Traditionally, the polymer production involved mixing the reactants, feed into a thermal reactor where reactants convert into polymer under heat, pressure and expensive catalysts. The polymer solid is separated off and cleaned, to be processed into polymer product, while the by-product is recovered as chemicals for sale.
elerGreen’s proprietary electrochemical process dramatically reduces the heat, pressure and catalyst requirements, and instead uses electricity that can come from carbon-free source. The electrochemical process allows parts or all of the materials to be replaced with chemical waste, where extra revenue can be generated out of the waste management service, while the recovery unit can be omitted to save cost when the by-product is benign, such as water.
The process was designed to be compatible with conventional polymer process, where the electrochemical reactor can readily replace the conventional reactor, to enhance reliability while allowing flexibility to retrofit outdated polymer facilities for reduced capital cost and enhanced profitability.
Technology

Historically, the idea of producing polymers electrochemically has always been a tempting for its nature of low cost and low environmental footprint but deemed a crazy idea and limited to a niche of conductive polymer. The reason is that when electrochemical reaction to function, the solid has to be conductive or it would quickly halt the reaction by blocking the conductivity as it forms on electrode surface.
elerGreen solved this electrode blockage problem, by an inventive step whereby the electrode is redesigned to be in relative motion against a removal blade, to scrubbed non-conductive polymers continuously from the electrode against blockage. The solid removal is also more efficient than conventional robotic or manual electrode stripping method, that has application for electrometallurgy.
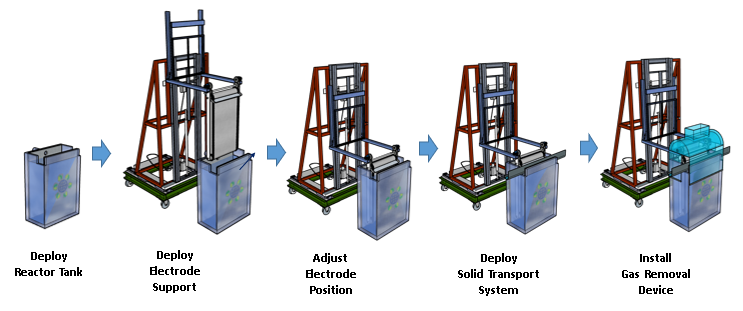
The technology has been optimized at multiple scales, including the modular reactor system designed for ease of manufacturing, deployment and scale-up, where the parts can be disassembled and reassembled:
Opportunities
The business potential lies with the cost savings from cheaper materials and lower energy cost, with hedging via diversified revenue streams, where risks are further minimized by process retrofit features reducing cost yet enhancing reliability.
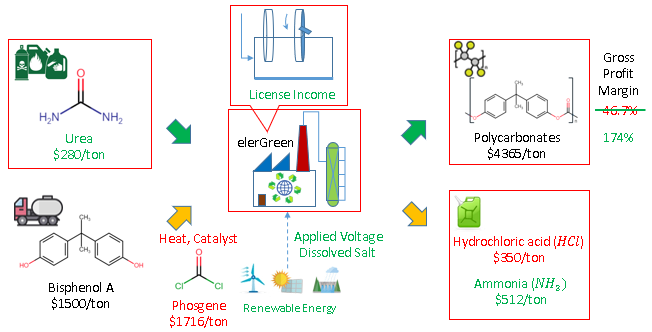
As demonstrative example, polycarbonates are conventionally produced from Bisphenol A and Phosgene, under heat from fossil fuel combustion and expensive catalysts, with hydrochloric acid as the saleable feedstock. The electrochemical route produces the same polycarbonates, where alternative materials can be used such as urea, under applied voltage from renewable electricity and dissolved salt, allows the use of urea as chemical or agricultural wastes. The replacing to the lower cost materials of urea result in ammonia instead of hydrochloric acid, but the same polycarbonate can be sold as the economic backbone of the process.
Just by replacing the core reactor unit from conventional to electrochemical, the gross profit margin more than triples, from 47% to 174%, and it has not taken account of the energy savings of around 80%, as well as the reduction in capital cost from equipment and catalyst. The utilization of carbon dioxide comes from urea, a more active derivative of carbon dioxide, where the conversion of carbon dioxide to urea has been a mature chemical process. Urea can also be obtained as biomass waste to stabilize supply source by diversity.
Furthermore, the polycarbonate reaction, cited for concept illustration is one out of the more than 100 polymers possible depending on starting materials. This ensures diversified revenue against fluctuation in single polymer market. The revenue source is further diversified beyond Polymer industry, to selling chemical by-products, waste management service and solid removal reactor with market in electrometallurgy industry.
Press the Next Button to move to the actual survey.
Note that it would open in new windows so that you can refer to this page when thinking about your response.
